A diverse group of aromatic substances. Charles Henschen, 2015.
Norisoprenoids are a diverse class of aromatic compounds that contribute to the varietal character of many wines, especially in aromatic varieties such as Riesling. Among the most important norisoprenoids for wine character are ß-Damascenone, 1,1,6,-trimethyl-1,2-dihydronapthalene (TDN), and vitispirane. ß-ionone, actinidiol, 3-oxo-α-ionol, and 2,2,6-trimethylcyclohexenone are other members of this class that are found in wine (Figure 1). ß-Damascenone has a pleasant, fruity aroma and seems to act as an “aroma enhancer”, boosting the intensity of other fruity-smelling compounds. TDN is responsible for the “petrol” or “kerosene” aroma typically found in aged Rieslings, and likely contributes to the complexity of other wines as well. Vitispirane has an aroma that has been described as floral, fruity, woody, or reminiscent of eucalyptus. Norisoprenoids are detectable at very low levels: sensory thresholds have been reported as low as 2 ng/L, although it is more likely that they begin to impact wine character at levels between 2 and 10 µg/L. Their concentration in wine tends to fall close to these threshold values, although levels of TDN in aged Riesling can reach 50+ µg/L.
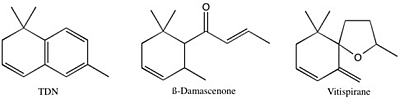
Figure 1. Structures of three major C13-norisoprenoids found in wine.
Norisoprenoids originate in large carotenoid molecules found in grapes, such as ß-carotene and lutein. These compounds accumulate during ripening, but break down into smaller compounds as the grapes reach maturity. At the beginning of the winemaking process, these compounds are bound to sugars, rendering them aromatically inactive. But during fermentation and as wine ages, they are released from the sugars via hydrolysis and eventually develop into the aromatic norisoprenoids. ß-damascenone seems to form primarily during fermentation, while TDN and vitispirane tend to reach their highest concentrations after extended aging. In the vineyard, increased sunlight exposure to the grapes seems to encourage the development of carotenoids, and subsequently increase the levels of norisoprenoids in finished wine. This effect probably occurs because the carotenoids help protect the grape tissue from ultraviolet light. In the laboratory, norisoprenoids are most often measured by gas chromatography-mass-spectroscopy.
References:
Eggers, N.J., Bohna, K., and B. Dooley. 2006. Determination of vitispirane in wines by stable isotope dilution assay. Am. J. Enol. Vitic. 57(2):226-232.
Mendes-Pinto, M.M. 2009. Carotenoid breakdown products the—norisoprenoids—in wine aroma. Arch. Biochem. Biophys. 483:236-245.
Sacks, G.L., Gates, M.J., Ferry, F.X., Lavin, E.H., Kurtz, A.J., and T.E. Acree. 2012. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 60:2998-3004.
Sefton, M.A., Skouroumounis, G.K., Elsey, G.M., and D.K. Taylor. 2011. Occurrence, sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 59:9717-9746.