Andrew Waterhouse
Department of Viticulture & Enology
University of California, Davis
This method is used routinely in our lab to measure total phenol. The procedure is also used for analysis of total phenol in tea. It uses the minimum volume of reagents and almost eliminates wasted reagent. Good micro pipets must be used for reproducibility. Plastic or glass cuvettes can be used. It is based on the method reported in Slinkard, K.; Singleton, V. L. Total Phenol Analysis: Automation and Comparison with Manual Methods. American Journal of Enology and Viticulture 1977, 28: 49-55; only the volumes have been reduced. If you cannot reproducibly measure such small volumes, try to reduce the volumes to the smallest you can. This reduces waste and disposal volume.
Folin Ciocalteu Reagent. This is usually purchased as the 2N reagent available from Sigma (F9252) or from Fisher Scientific (ICN19518690), and presumably others. Singleton and Rossi AJEV 1965, 16: 144-158, describe the preparation of the reagent from sodium tungstate, sodium molybdate, lithium sulfate, bromine, and some acids. Solutions containing the FC reagent must be disposed of as hazardous waste.
Gallic Acid Stock Solution. In a 100-mL volumetric flask, dissolve 0.500 g of dry gallic acid in10 mL of ethanol and dilute to volume with water. Can be opened daily, but to store, keep closed in a refrigerator up to two weeks.
Sodium Carbonate Solution. Dissolve 200 g of anhydrous sodium carbonate in 800 mL of water and bring to a boil. After cooling, add a few crystals of sodium carbonate, and after 24 hr, filter and add water to 1 L. Should be stable indefinitely.
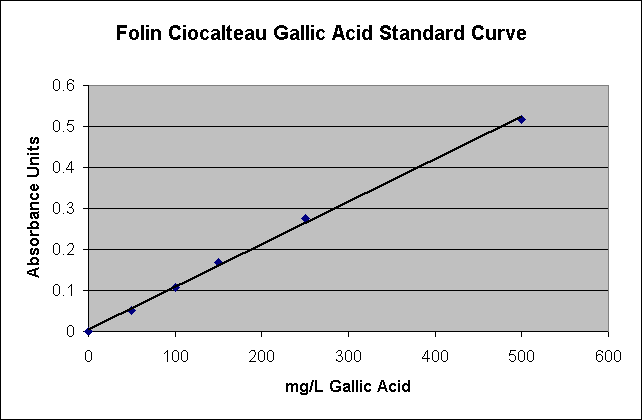
To prepare a calibration curve, add 0, 1, 2, 3, 5, and 10 mL of the above phenol stock solution into 100 mL volumetric flasks, and then dilute to volume with water. These solutions will have phenol concentrations of 0, 50, 100, 150, 250, and 500 mg/L gallic acid, the effective range of the assay. Left over gallic acid solutions can be poured down the drain.
From each calibration solution, sample, or blank, pipet 20 µL into separate cuvettes, and to each add 1.58 mL water, and then add 100 µL of the Folin-Ciocalteu reagent, and mix well. Wait for between 30 sec and 8 min, and then add 300 µL of the sodium carbonate solution , and shake to mix. Leave the solutions at 20°C for 2 hr and determine the absorbance of each solution at 765 nm against the blank (the "0 mL" solution) and plot absorbance vs. concentration. Alternatively, they can be left at 40°C for 30 min before reading the absorbance.
For white wines, add 20 µL as for the calibration solutions, but in the case of red wines, dilute the wines by 10 first, then add 20 µL (or skip the dilution and add 2 µL if you have precise micro pipettors).
Create a calibration curve with the standards and determine the levels in the samples. Do not neglect to multiply the observed concentrations by any dilution factor of the original sample. Results are reported at Gallic Acid Equivalent, GAE, because the phenols in wine contain mostly other phenols, and only small amounts of gallic acid.
Interferences. Reducing sugars such as glucose and fructose cause minor interferences and must be corrected. Sulfites also cause an interference but the magnitude is variable. It is usually not an important factor except for white wines with medium to high sulfite levels (>50 mg/L) and low phenol levels (<250 mg/L). Ascorbate is another interference. It has a relative mass response of 0.68, so a wine with 30 mg/L ascorbate must be corrected by subtracting 20.4 mg/L from the total phenol value. This assay is responsive to any reducing substance, so if applied to other types of samples, large errors could be encountered.
Since the assay measures all phenolics, the choice of gallic acid as standard is based on the availability of a stable and pure substance, and gallic acid is both, and it is less expensive than other options. In addition, the response to gallic acid has been shown to be equivalent to most other phenolics in wine on a mass basis. We have also tested the stability of gallic acid standard solutions and we can say they lose less than 5% of their value over two weeks when refrigerated and kept tightly closed.
You MUST make up your own standard curve each time the analysis is run. Do not use this data as a standard curve. It is here only as an example of a typical standard curve.
For more details on the Folin Ciocalteau analysis procedure, please see: Waterhouse, A.L., Determination of Total Phenolics, in Current Protocols in Food Analytical Chemistry, I1.1.1-I1.1.8, Wrolstad, R.E., Wiley, 2001, or Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152-178.